A 510(k) cleared delivery device to address the therapeutic challenge.
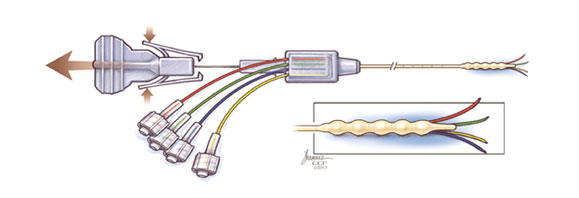
Key Features
- 510(k) Cleared
- Delivery via four independent, flexible micro-catheters
- Proprietary anti-backflow design
- Applicable within any operating room (OR) with use of conventional neuronavigation
- Ability to remain in place and be used for multiple days (enabling large distribution volumes)
- Removable at bedside
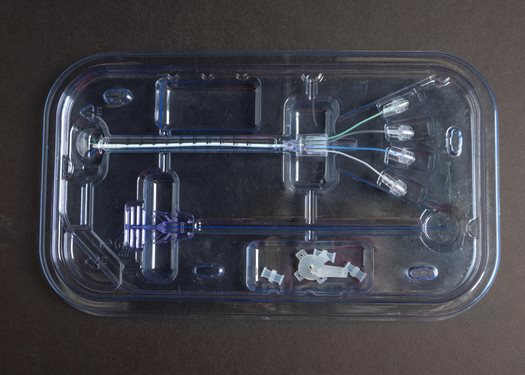
Facts & Figures
Dimensions
Catheter: Length 14 cm; 2.5 mm diameter
Microcatheters: Four .38 mm diameter
Connectors: Four Male Luer
Overall Length including Stylet: 22.2 cm
Overall Length without Stylet: 19.3 cm
Extended Length: 18.5 cm (catheter tip to back of housing)
Relaxed Length: 17.3 cm (catheter tip to back of housing)
Materials
Silicone Catheter
Teflon Microcatheters
Thermoplastic Housing and Luers
MRI Compatible Stainless Steel Stylet
Silicone Suture Tab
Patents
US Patents #8,808,234, #8,979,822
Notes: The Cleveland Multiport Catheter is an investigational device and can be used only in the setting of an FDA-approved clinical trial. The purpose of this website is only to obtain investigators and not to make the device generally available.